PHIP (Parahydrogen-Induced Polarization) +
We place great emphasis on maximum transparency from the very beginning. Since our contrast agent is injected into the body of the patients, it is only natural for us to fully disclose its structure and properties. This way, everyone can understand how our contrast agent works and that it is completely safe.
As already explained here, we use a signal enhancement method called PHIP (Parahydrogen-Induced Polarization). A prerequisite for a molecule to be hyperpolarized using PHIP is the presence of an unsaturated bond. Such bonds occur naturally and play important roles in the human body—such as in the form of unsaturated fatty acids.
From the start, we were interested in using pyruvate as a contrast agent—but why? Pyruvate is the ideal metabolite for imaging metabolism. It is a particularly biologically active form of sugar: When the human body absorbs glucose, it splits it into two pyruvate molecules, which are then introduced into the citric acid cycle—the central pathway for energy production.
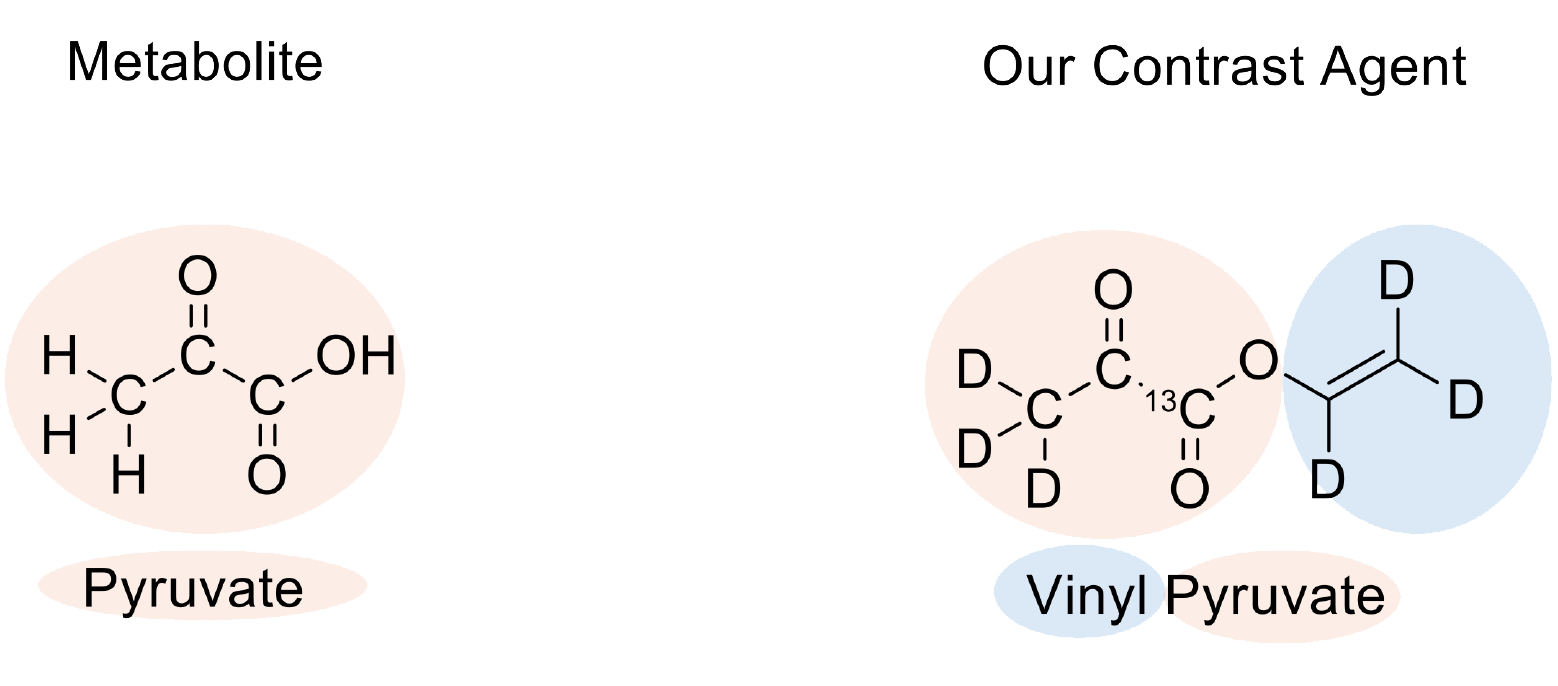
The problem: Pyruvate does not contain an unsaturated bond and therefore cannot be directly hyperpolarized using the PHIP method.
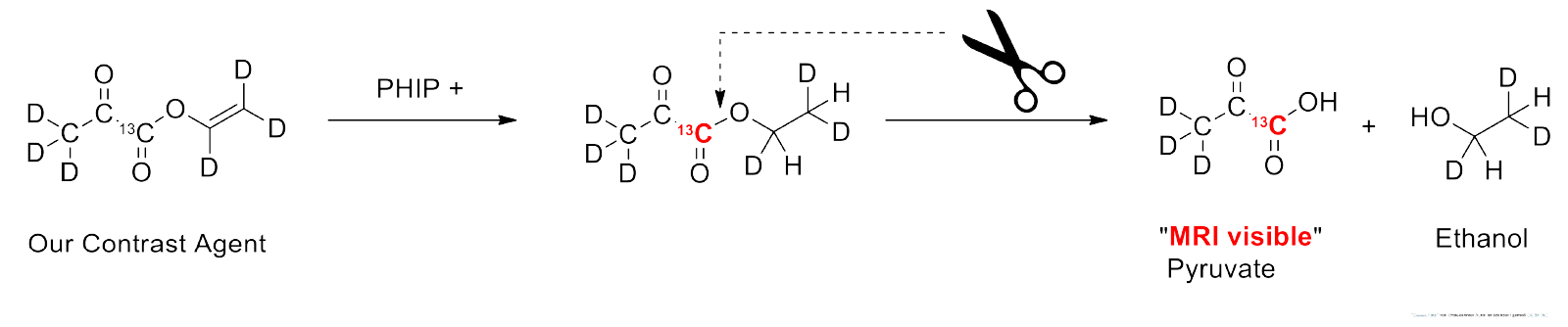
To overcome this obstacle, we added a so-called unsaturated side arm to our pyruvate molecule. This side arm can absorb parahydrogen, allowing us to utilize the resulting spin order to generate hyperpolarization in the pyruvate part of the molecule.
The great advantage of this side arm: It can be quickly detached after hyperpolarization. What remains is the desired metabolite, pyruvate—now in its hyperpolarized form.
This principle is not new in itself, as it was already published in 2014. The problem until now: the unsaturated side arm cannot have just any structure if excellent results are to be achieved. If the double bond of the side arm is further away from the pyruvate part, the polarization can be transferred less effectively.. The shortest unsaturated side arm is the so-called vinyl side arm. However, this has not been able to be attached to pyruvate until now. Several research groups have unsuccessfully attempted to develop an efficient synthesis for years. The best yields in the production of vinylpyruvate (the name for the entire molecule) in their case were just 6-8%.
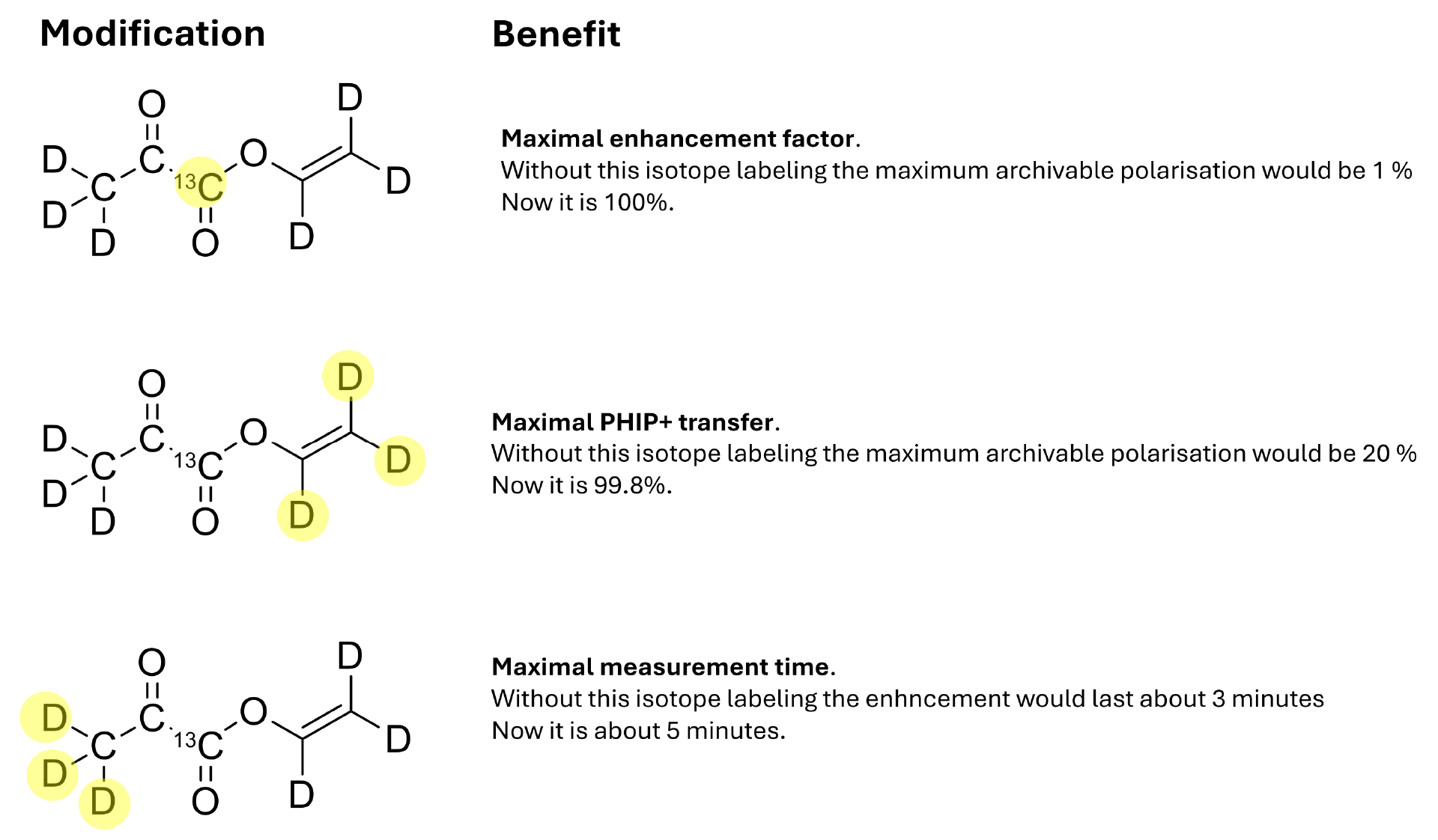
If you want truly exceptional signal enhancement, the vinyl arm alone is not sufficient. To achieve this goal, the side arm and the metabolite must be labeled with isotopes. Isotope labeling makes the synthesis even more difficult, so the best synthesis route to isotopically labeled vinylpyruvate that the research groups could use resulted in only a 3% yield.
Recently, we made a breakthrough in the synthesis of vinylpyruvate. With our specially developed and patented synthesis method for this purpose, we were able to produce vinylpyruvate with a yield of up to 70%. The completely isotopically labeled vinylpyruvate was also produced with yields of up to 50%. Compared to existing methods, this represents an improvement of almost 1700%.
It is only through our synthetic breakthrough that it is now possible to perform imaging with highly polarized pyruvate.