Our New Contrast Agent
We place great emphasis on maximum transparency from the very beginning. Since our contrast agent is injected into the body of the patients, it is only natural for us to fully disclose its structure and properties. This way, everyone can understand how our contrast agent works and that it is completely safe.
As already explained here [LINK], we use a signal enhancement method called PHIP (Parahydrogen-Induced Polarization). A prerequisite for a molecule to be hyperpolarized using PHIP is the presence of an unsaturated bond. Such bonds occur naturally and play important roles in the human body—such as in the form of unsaturated fatty acids.
From the start, we were interested in using pyruvate as a contrast agent—but why? Pyruvate is the ideal metabolite for imaging metabolism. It is a particularly biologically active form of sugar: When the human body absorbs glucose, it splits it into two pyruvate molecules, which are then introduced into the citric acid cycle—the central pathway for energy production.
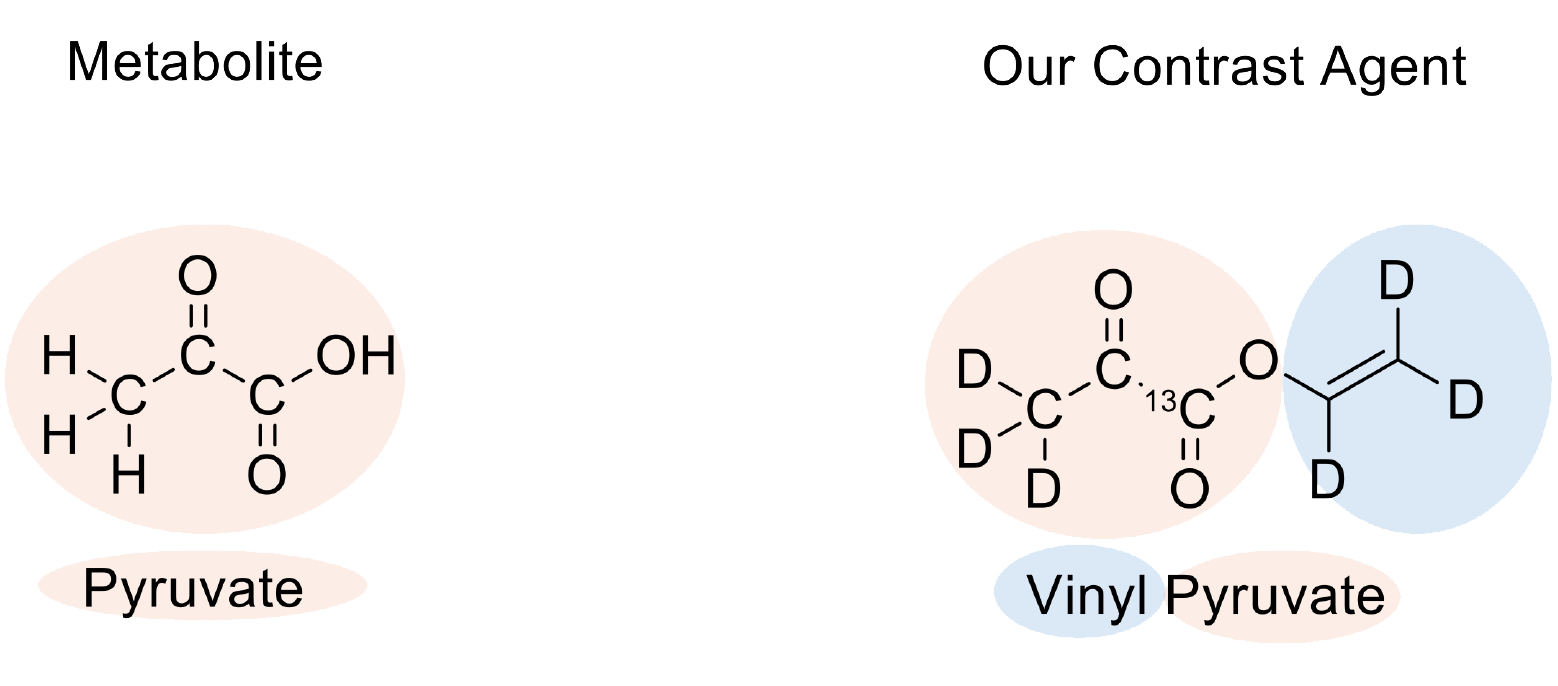
The problem: Pyruvate does not contain an unsaturated bond and therefore cannot be directly hyperpolarized using the PHIP method.
To overcome this obstacle, we added a so-called unsaturated side arm to our pyruvate molecule. This side arm can absorb parahydrogen, allowing us to utilize the resulting spin order to generate hyperpolarization in the pyruvate part of the molecule.
The great advantage of this side arm: It can be quickly detached after hyperpolarization. What remains is the desired metabolite, pyruvate—now in its hyperpolarized form.