Revolutionizing MRI Technology with Exceptional Signal Amplification via PHIP +
How Does Our PHIP+ Process Work?
In scientific terms: We have developed a precursor molecule—a pre-stage of the metabolite—that contains an unsaturated side arm in addition to the metabolite pyruvate. This precursor can be hydrogenated with inexpensive para-hydrogen in the side arm, and the high spin order of the transferred hydrogen protons can be utilized to create hyperpolarization in the ¹³C nucleus of the pyruvate through a targeted spin order transfer. The relatively long half-life of the hyperpolarization in this ¹³C nucleus then allows the side arm of the precursor compound to be cleaved off, isolating the hyperpolarized pyruvate for injection.
But how exactly does our PHIP+ process work? How can we hyperpolarize endogenous metabolites?
If you're not a physicist or chemist, many of these terms may not mean much to you. So here’s a more detailed explanation to help you better understand the advantages of our process and, in particular, our patented contrast agent.
Some atomic nuclei, such as hydrogen nuclei or ¹³C nuclei, behave like tiny magnets in a magnetic field and align themselves accordingly. In MRI, the behavior of the overall "magnetization" generated by all these atomic mini-magnets is used to create an image. (Technically, it is the induced electric current that are measured.)
The issue is that almost half of these atomic magnets behave exactly opposite to the other half, effectively canceling each other out—almost. A small fraction of the magnets remains uncanceled, creating a measurable signal. Only this weak signal from the excess magnetization contributes to the final MRI image. This excess magnetization is also often referred to as polarization.
However, the number of atoms that contribute to this excess magnetization (or polarization) is extremely low in conventional MRI. In a typical MRI scan, only about 1 in 1 million atomic nuclei contribute to the measurable signal—the remaining 999 999 atoms are invisible to the MRI. This is why only very abundant atomic nuclei, such as protons (since the human body is composed of about 80% water, with each water molecule containing two protons), can generate a signal strong enough to be detected in a reasonable timeframe.
To deal with this weak signal in conventional MRI, there are two main strategies:
- Increase the magnetic field strength – This is extremely expensive and has physical limitations.EG you would need the magnetic field of a neutron star if you wanted to create a with conventional mri as with hyperpolarized MRI. Humanity has a long way to go to create such powers, ist litterally science fiction right now.
- Repeat the measurement multiple times and sum the signals – This improves signal-to-noise ratio but requires taking multiple images and create an overlay. Thios increases the scan time. Moreover qick and dynmaic processes in the body, like metabolsim can never be detectet using this approach because each image woul differ from the previous one, making an overlay impssible.
To illustrate the sensitivity problem in conventional MRI, imagine that the excess magnetization represents water in a 1-liter beaker. If the beaker is empty, all spins cancel each other out completely—there is no measurable MRI signal. If the beaker is completely full, all mini-magnets align in one direction, and their combined magnetic fields create the strongest possible MRI signal.
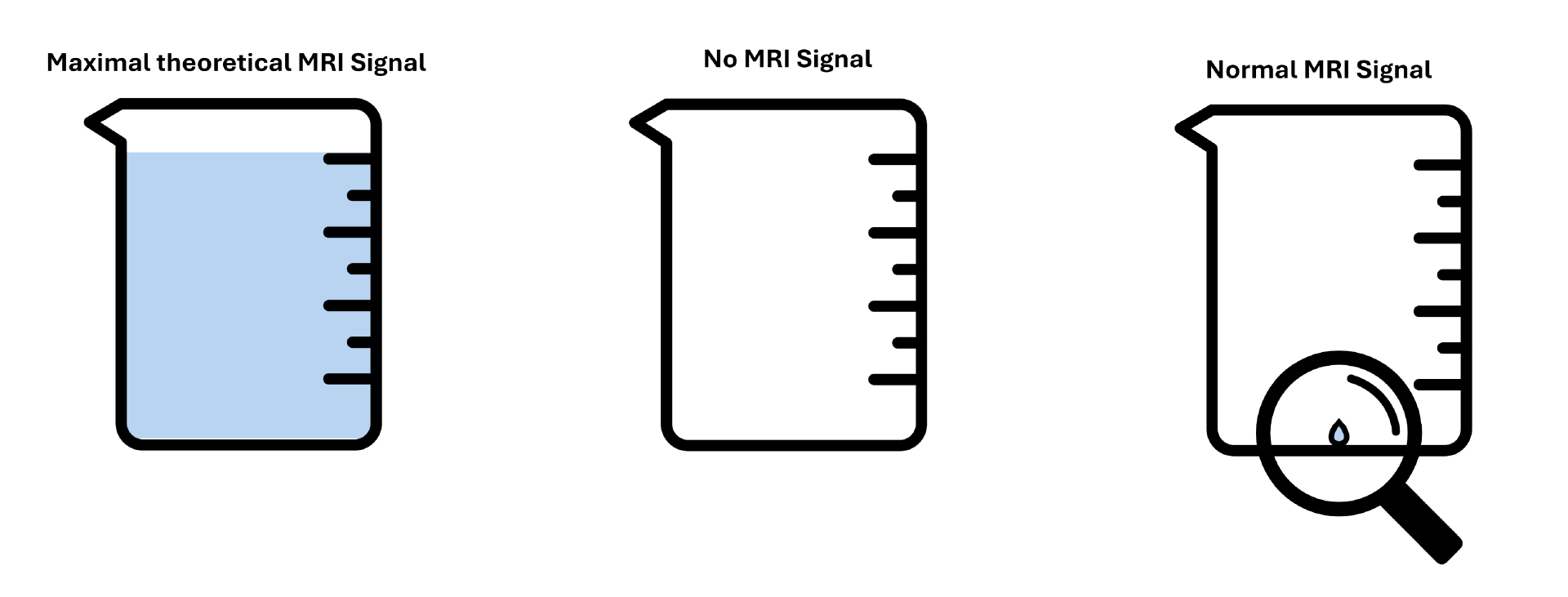
In standard MRI, however, the beaker is barely filled—with about one microliter of water. Yes, you read that correctly: not a milliliter, but a microliter—roughly 1,000 times smaller than a droplet. This tiny signal is what clinical MRI relies on to generate black-and-white images of your organs. Because the signal is so weak, only the most abundant atoms in the human body—hydrogen nuclei—can be detected. These include hydrogen atoms in water, fats, carbohydrates, and proteins, which make up different tissue types.
As mentioned earlier, multiple images must be taken and overlaid to produce the final image you see in clinical MRI. This is one reason why some MRI scans take a long time, requiring patients to remain still—otherwise, misalignment between scans could cause blurring.
What Does Hyperpolarization Do?
In simple words: Hyperpolarization fills the beaker by more than just the tiny droplett—meaning it amplifies the measurable MRI signal.
Sounds simple, right? In reality, it’s quite complex.
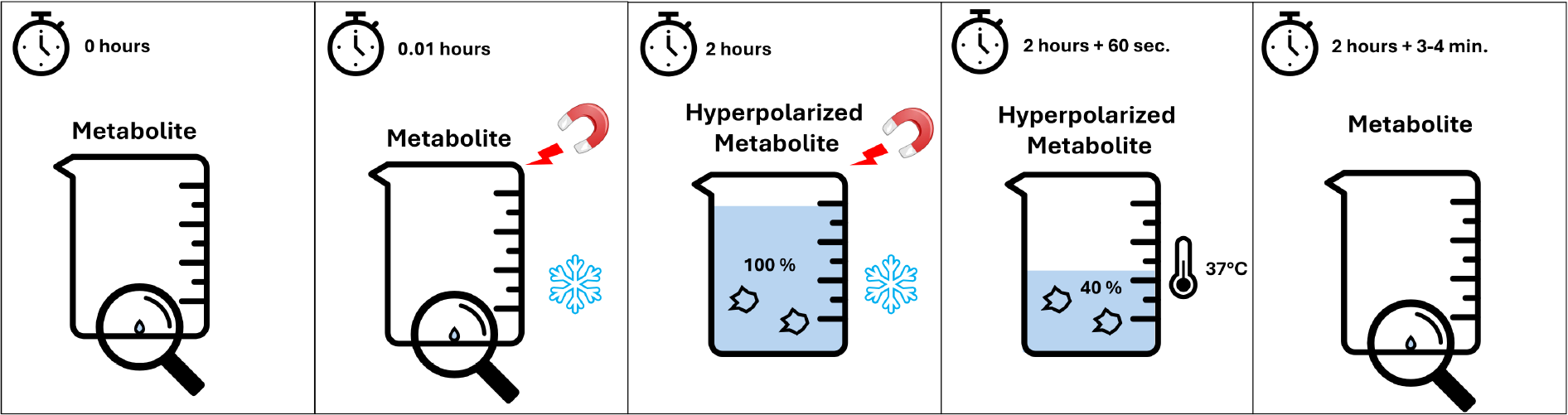
If we were to directly fill the beaker (increase the measurable MRI signal) of our contrast agent, extreme conditions would typically be required. The most widely used clinical research method, D-DNP (Dynamic Nuclear Polarization), relies on extremely strong magnetic fields and ultra-low temperatures near absolute zero (very, very cold… and very, very expensive) to hyperpolarize small amounts of a metabolite just before the measurement. This method is cumbersome, costly, and time-consuming (taking several hours per measurement, just for preparation).
Another challenge: If we fill the beaker beyond the normal microliter level, it quickly "leaks". Within 3 to 5 minutes, the extra signal is lost, returning to its original weak state. In physics, this is known as relaxation, meaning the system naturally returns to equilibrium (an almost empty beaker—almost no signal intensity).
At first glance, this seems like a bad deal: a highly complex process that significantly enhances the MRI signal but is expensive and short-lived.
We Do It Better
However, we use clever chemical and physical tricks to achieve high signal enhancement quickly and affordably, while ensuring the hyperpolarized contrast agent remains detectable long enough for a high-quality MRI scan.

First, instead of hyperpolarizing the metabolite directly, we use an unsaturated side arm. This side arm also has a 1-liter beaker.
We hydrogenate this side arm with para-hydrogen, a special quantum form of hydrogen. This is equivalent to filling the beaker of the side arm under a running tap until it’s full. The huge advantage of this approach is that it is incredibly fast (seconds!), uses cheap and widely available para-hydrogen, and the para-hydrogen can be stored for months. Essentially, para-hydrogen serves as a perfect water reservoir for us (in reality, a highly accessible and cost-effective source of spin order).
After filling the side arm’s beaker, we need to act quickly because this beaker is also leaky—very, very leaky. We transfer the water from the side arm’s beaker into the contrast agent’s beaker. Unfortunately, some water spills during the transfer, and both beakers continue to leak.
Our competitors, who use a similar approach but with a different contrast agent, typically achieve a fill level of around 200 ml in the contrast agent’s beaker. That’s impressive—but we can do better.
With our patented contrast agent, which is isotope-labeled in both the metabolite and the side arm, we reduce the number of leaks in both beakers. Additionally, we use a special vinyl side arm, which shortens the transfer distance—reducing spillage.
As a result, we have successfully demonstrated higher signal retention. Our improvements have already enabled us to reach fill levels of around 300 ml in the contrast agent’s beaker. Calculations suggest that by speeding up the transfer process and further automating it, we could achieve 600–700 ml.
Furthermore, about one-third of the leaks in the contrast agent’s beaker have been sealed, ensuring that the hyperpolarization lasts longer, allowing for higher signal strength and extended measurement times.